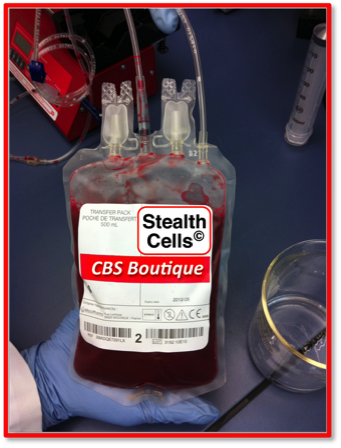
Immunocamouflaged RBC
(Stealth RBC)
Alloimmunization to non-ABO Blood Group Antigens is a significant problem in transfusion medicine - especially in the chronically transfused patient. Our initial work on the immunocamouflage of cells was focused on this problem and led to the development of the "Stealth RBC". By grafting methoxy(Poly Ethylene) glycol to the membrane of normal donor RBC we camouflage the non-ABO antigens from immune recognition (ie., immunocamouflage) byt the blood recipient. This approach is being actively pursued within Canadian Blood Services as a means to address the transfusion needs of patients with severe alloimmunization or extremely rare blood types.
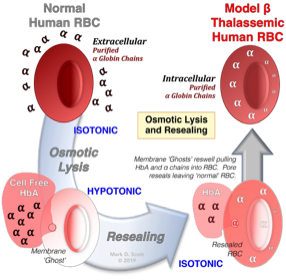
The Utility Of RBC Osmotic Lysis And Resealing In The Study Of Cellular Function And Dysfunction
Unravelling the role of superoxide dismutase, catalase, glutathione and NADPH in the oxidant defense of human and mouse RBC.
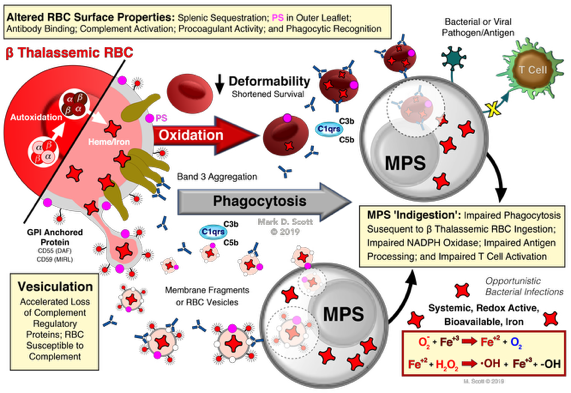
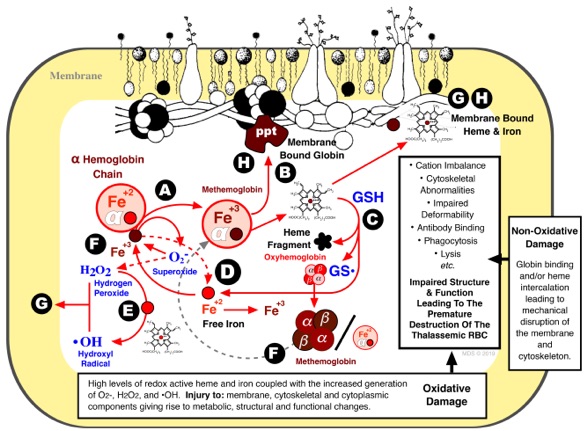
Modeling human β thalassemic RBC to elucidate the mechanism(s) underlying the destruction of the thalassemic cell.

Relevant Laboratory Publications
Scott, M.D., Meshnick, S.R., and Eaton, J.W. Superoxide dismutase-rich bacteria: Paradoxical increase in oxidant toxicity. J. Biol. Chem. 262:3640-3645(1987). PMID: 3546314
Scott, M.D., Meshnick, S.R., and Eaton, J.W. Superoxide dismutase amplifies organismal sensitivity to ionizing radiation. J. Biol. Chem. 264:2498-2501(1989). PMID: 2644263
Bütikofer, P., Lin, Z.W., Kuypers, F.A., Scott, M.D., Xu, C., Wagner, G.M., Chiu, D.T.-Y., and Lubin, B. Chlorpromazine inhibits vesiculation, alters phosphoinositide turnover and improves deformability of ATP-depleted red blood cells. Blood 73:1699-1704(1989). PMID: 2540856
Scott, M.D., Meshnick, S.R., Williams, R.A., Chiu, D.T.-Y., Pan, H.C., Lubin, B.H., and Kuypers, F.A. Qinghaosu-mediated oxidation in normal and abnormal erythrocytes. J. Lab. Clin. Med. 114:401-406(1989). PMID: 2794752
Scott, M.D., Eaton, J.W., Chiu, D. T.-Y., Kuypers, F.A., and Lubin, B.H. Enhancement of erythrocyte superoxide dismutase activity: Effect on cellular oxidant defense. Blood 74:2542-2549(1989). PMID: 2553167
Scott, M.D., Kuypers, F.A., Bütikofer, P., Bookchin, R.M., Ortiz, O., and Lubin, B.H. Effect of osmotic lysis and resealing on red cell structure and function. J. Lab. Clin. Med. 115:470-480(1990). PMID: 1691257
Meshnick, S.R., Scott, M.D., Lubin, B.H., Ranz, A. and Eaton, J.W. The antimalarial activity of diethyldithiocarbamate: Potentiation by copper. Biochemical. Pharmacology. 40:213-216(1990). DOI: https://doi.org/10.1016/0006-2952(90)90680-j
Scott, M.D., Ranz, A., Kuypers, F.A., Lubin, B.H., and Meshnick, S.R. Parasite uptake of desferroxamine: A prerequisite for antimalarial activity. Br. J. Haematol. 75:598-602(1990). DOI: https://doi.org/10.1111/j.1365-2141.1990.tb07805.x
Rouyer-Fessard, P., Scott, M.D., Garel, M.C., Bachir, D., Galacteros, F., and Beuzard, Y. Fate of α-hemoglobin chains and erythrocyte defects in β Thalassemia. in Sixth Cooley’s Anemia Symposium (Bank, A. ed.) Annals of the New York Academy of Sciences, 612:106-117(1990). DOI: https://doi.org/10.1111/j.1749-6632.1990.tb24296.x
Scott, M.D., Rouyer-Fessard, P., Lubin, B.H., and Beuzard, Y. Entrapment of purified α-hemoglobin chains in normal erythrocytes: A model for β thalassemia. J. Biol. Chem. 265:17953-17959(1990). PMID: 2211672
Kuypers, F.A., Scott, M.D., Schott, M.A., Chiu, D.T.-Y., and Lubin, B.H. Use of ektacytometry to assess red cell susceptibility to oxidative stress. J. Lab. Clin. Med. 116:535-545(1990). PMID: 2212862
Scott, M.D., Zuo, L., Lubin, B.H., and Chiu, D.T.-Y. NADPH, not glutathione, status modulates oxidant susceptibility of hemoglobin in normal and glucose-6-phosphate dehydrogenase deficient erythrocytes. Blood, 77:2059-2064(1991). PMID: 2018843
Scott, M.D., Lubin, B.H., Zuo, L., and Kuypers, F.A. Erythrocyte defense against H2O2: Preeminent importance of catalase. J. Lab. Clin. Med., 118:7-16(1991). PMID: 2066646
Scott, M.D., Rouyer-Fessard, P., Ba, M.S., Lubin, B.H., and Beuzard, Y. α- and β-hemoglobin chain induced changes in normal erythrocyte deformability: Comparison to β thalassemia intermedia and Hb H disease. Br. J. Haem., 80:519-526(1992). DOI: https://doi.org/10.1111/j.1365-2141.1992.tb04567.x
Hong, Y.-L., Pan, H.-Z., Scott, M.D., and Meshnick, S.R. Activated oxygen generation by a primaquine metabolite: Inhibition by antioxidants derived from Chinese herbal remedies. Free Rad. Biol. Med., 12:213-218(1992). DOI: https://doi.org/10.1016/0891-5849(92)90029-g
Leroy-Viard, K., Royer-Fessard, P., Sauvage, C., Scott, M.D., and Beuzard, Y. Modéles expérimentaux de la ß-thalassémie (Experimental models for ß-Thalassemia). Medicinè/Science, 8:784-789(1992). DOI: https://doi.org/10.4267/10608/3228
Lubin, B.H., Cahn, S., and Scott, M.D. Hematologic manifestations, in: Biomedical Concerns in Persons with Down Syndrome (Pueschel, S.M. and Pueschel, J.K., eds.). Paul Brookes Publishing Co., Baltimore, 223-257(1992). DOI: https://doi.org/10.1007/978-1-4615-3030-5_17
Scott, M.D. Entrapment of purified α-hemoglobin chains in normal erythrocytes as a model of human β thalassemia. in: The Use of Resealed Erythrocytes as Carriers and Bioreactors. (Magnani, M. and DeLoach, J.R., eds.) Plenum, New York, pgs. 139-148(1992). ISBN: 0-306-44345-7
Scott, M.D., Wagner, T.C., and Chiu, D.T.-Y. Decreased catalase activity is the underlying mechanism of oxidant susceptibility in glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim. Biophys. Acta, 1181:163-168(1993). DOI: https://doi.org/10.1016/0925-4439(93)90106-b
Minetti, M., Mallozzi, C., Scorza, G., Scott, M.D., Kuypers, F.A., and Lubin, B.H. Role of oxygen and carbon radicals in hemoglobin oxidation. Arch. Biochem. Biophys., 302:233-244(1993). DOI: https://doi.org/10.1006/abbi.1993.1205
Scott, M.D., van den Berg, J.J.M., Repka, T., Rouyer-Fessard, P., Hebbel, R.P., Beuzard, Y., and Lubin, B.H. Effect of excess α-hemoglobin chains on cellular and membrane oxidation in model ß thalassemic erythrocytes. J. Clin. Invest., 91:1706-1712(1993). DOI: https://doi.org/10.1172/jci116380
Lubin, B.H., van den Berg, J.J.M., Lewis, R.A., Scott, M.D., and Kuypers, F.A. Unique properties of the neonatal red cell. In: Neonatal Immunology and Haematology II (Xanthou, X., Bracci, R., and Prindull, G., eds.) Elsevier Science Publishers, Amsterdam., pgs. 79-89(1993). ISBN: 9780444816566
Lopez-Shirley, K., Zhang, F., Gosser, D., Scott, M.D. and Meshnick, S.R. Antimalarial quinones: Redox potential dependence of methemoglobin formation and heme release in erythrocytes. J. Lab. Clin. Med. 123:126-130(1994). PMID: 8288952
Wagner, T.C. and Scott, M.D. Single extraction method for the spectrophotometric quantification of oxidized and reduced pyridine nucleotides in erythrocytes. Anal. Biochem., 222:417-426(1994). DOI: https://doi.org/10.1006/abio.1994.1511
Scott, M.D. Glucose-6-phosphate dehydrogenase deficiency: A new hypothesis for an old disease. Redox Report, 1:235-237(1995). DOI: https://doi.org/10.1080/13510002.1995.11746992
Salas, F., Fichmann, J., Lee, C.K., Scott, M.D., and Rosenthal, P.J. Functional expression of falcipain, a Plasmodium falciparum cysteine proteinase, supports its role as a malarial hemoglobinase. Infect. Immun., 63:2120-2125(1995). PMID: 7768590
Scott, M.D. and Eaton, J.W. Thalassemic Erythrocytes: Cellular suicide arising from iron and glutathione-dependent oxidation reactions? Br. J. Haem., 91:811-819(1995). DOI: https://doi.org/10.1111/j.1365-2141.1995.tb05394.x
Kuypers, F.A., Schott, M.A. and Scott, M.D. Phospholipid composition in model ß thalassemic erythrocytes. Am. J. Hematol., 51:45-54(1996). DOI: https://doi.org/10.1002/(sici)1096-8652(199601)51:1%3C45::aid-ajh8%3E3.0.co;2-7
Eaton, J.W. and Scott, M.D. Cooperativity in oxidant defense. In: Nitric Oxide and Radicals in the Pulmonary Vasculature (Weir, E.K., Archer, S.L., and Reeves, J.T., eds) Futura Publishing Co., Inc. Armonk, NY pgs. 155-166(1996). ISBN: 9780879936310
Scott, M.D. and Eaton, J.W. Superoxide is not the proximate cause of paraquat toxicity. Redox Report, 2:113-119(1996). DOI: https://doi.org/10.1080/13510002.1996.11747037
Scott, M.D. and Eaton, J.W. Markers of Free Radical-Mediated Tissue Injury. In: Free Radical Toxicology (Wallace, K.B., ed) Taylor & Francis, Washington D.C., pgs. 401-420(1997). ISBN: 9781439805701 (Old: 1-56032-632-8)
Scott, M.D. and Eaton, J.W. Parasite-mediated progeria: A possible mechanism for antimalarial action of G-6-PD deficient erythrocytes. In: Adaptations to Malaria: The Interaction of Biology and Culture (Greene, L.S. and Danubio, M.E., eds) Gordon and Breach Publishers, Amsterdam, pgs. 89-102(1997). ISBN: 9789057005046
Scott, M.D., Yang, L., Ulrich, P. and Shupe T. Pharmacologic interception of heme: a potential therapeutic strategy for the treatment of ß thalassemia? Redox Report, 3:159-167(1997). DOI: https://doi.org/10.1080/13510002.1997.11747104
Scott, M.D. Intraerythrocytic iron chelation therapy: An alternative to blood transfusions? Hematology, 6:73-89(2001). DOI: https://doi.org/10.1080/10245332.2001.11746557
Scott, M.D. H2O2 injury in ß thalassemic erythrocytes: Protective role of catalase and the prooxidant effects of GSH. Free Radicals in Biology & Medicine, 40:1264-1272(2006). DOI: https://doi.org/10.1016/j.freeradbiomed.2005.11.017
Rossi N.A., Mustafa, I., Jackson, J.K., Burt, H.M., Horte, S.A., Scott, M.D., Kizhakkedathu, J.N. In vitro chelating, cytotoxicity, and blood compatibility of degradable poly(ethylene glycol)-based macromolecular iron chelators. Biomaterials, 30:638-648 (2009). DOI: https://doi.org/10.1016/j.biomaterials.2008.09.057
Kwan, J.M., Guo, Q., Kyluik-Price D.L., Ma, H. and Scott, M.D. Microfluidic Analysis of Cellular Deformability of Normal and Oxidatively-Damaged Red Blood Cells. American Journal of Hematology, 88:682–689 (2013). DOI: https://doi.org/10.1002/ajh.23476
Guo, Q., Duffy, S.P., Matthews, K., Santoso, A.T., Scott, M.D. and Ma, H. Microfluidic Analysis of Red Blood Cell Deformability. Journal of Biomechanics, 47:1767–1776 (2014). DOI: https://doi.org/10.1016/j.jbiomech.2014.03.038
Matthews, K., Myrand-Lapierre, M.-E., Ang, R.R., Duffy, S.P., Scott, M.D., and Ma, H. Microfluidic Deformability Analysis of the Red Cell Storage Lesion. Journal of Biomechanics, 48(15):4065-4072 (2015). DOI: https://doi.org/10.1016/j.jbiomech.2015.10.002
Matthews, K., Duffy, S.P., Myrand-Lapierre, M.-E., Ang, R.R., Li, L., Scott, M.D., and Ma, H. Microfluidic analysis of red blood cell deformability as a means to assess hemin-induced oxidative stress resulting from Plasmodium falciparum intraerythrocytic parasitism. Integrative Biology, 9(6):519-528 (2017). DOI: https://doi.org/10.1039/c7ib00039a
Mustafa, I., Nasrallah, G., Scott, M.D., Ahmad Al-Jamal, O.L., Salem, R., and Daas, S. Biocompatibility and Toxicity of Novel Iron Chelator Starch-Deferoxamine (S-DFO) Compared to Zinc Oxide Nanoparticles to Zebrafish Embryo: An Oxidative Stress-based Apoptosis, Physicochemical and Neurological Study Profile. Neurotoxicology and Teratology, 72:29-38 (2019). DOI: https://doi.org/10.1016/j.ntt.2019.01.004
Scott, M.D. Model Human ß Thalassemic Erythrocytes: Effect of unpaired purified α-hemoglobin chains on normal erythrocytes. In: Beta Thalassemia (Editor: Zakaria, M.), INTECH. ISBN: 978-1-83880-587-6 (2019). in press. DOI: 10.5772/intechopen.90288
Red Blood Cell Oxidant Defense and the Use of Resealed Erythrocytes For The Study Of RBC Abnormalities
(e.g., The Model Thalassemic RBC)
Beginning in the late 1980’s my laboratory developed the “resealed” erythrocyte as an experimental tool for the analysis of red cell hemoglobinopathies, enzymopathies, and for the study of malaria. This procedure involves the use of osmotic lysis and resealing of erythrocytes for the purpose of incorporating agents within the red cell cytoplasm. Importantly, following this procedure, control-resealed red cells demonstrate normal structure and function and exhibit normal in vivo survival in animal models.
My studies demonstrated the utility of the cells for the investigation of thalassemia and glucose-6-phosphate dehydrogenase deficiency in human red blood cells. Additionally we have utilized these cells for the investigation of malaria and anti-malarial drugs and as drug carriers.


